What is radioactivity?

By Dr. Nick Touran, Ph.D., P.E., 2009-02-01, Updated 2020-02-22, Reading time: 9 minutes

Radioactive atoms contain energy that pours out spontaneously as energetic subatomic particles or electromagnetic waves. The emissions are called radiation. Radioactive material exists naturally in the Earth (this is partially why the inside of the Earth is warm) and is produced continuously in the atmosphere by cosmic rays. Humans make radioactive material by causing nuclear reactions in nuclear reactors and particle accelerators.
Like tiny bullets, the radiation from radioactive materials can cause damage to biological cells by breaking fragile biological structures such as DNA. Radiation can be used medically to kill cancer cells or in diagnostics. It can also be a hazard associated with nuclear technology.
Some radioactive materials pour their energy out quickly, and others pour it out slowly. The rate of energy release is quantified through the material’s half-life, which is the time after which half of the initial atoms have released their energy. For example, if you start with 100 radioactive atoms with a half-life of 1 minute, 50 of them will have emitted their energy after 1 minute. After 2 minutes, there will be 25 left, and so on.

Materials with shorter half-lives are the most dangerous, since they are ‘shooting out bullets’ rapidly and can cause faster cell damage. Fortunately, they naturally decay to stability faster. Materials with longer half-live cause less rapid cell damage but are around longer.
Many materials created during the operation of a reactor are radioactive. Radiation is the primary cause of both the waste and safety concerns related to nuclear energy.
The amount of cell damage radiation can do is proportional to the energy transfered to the cells. This is quantified by radioactive dose. Measurable dose rates span many orders of magnitude, from detectable but harmless to deadly. The ranges are summarized by the following DOE figure:
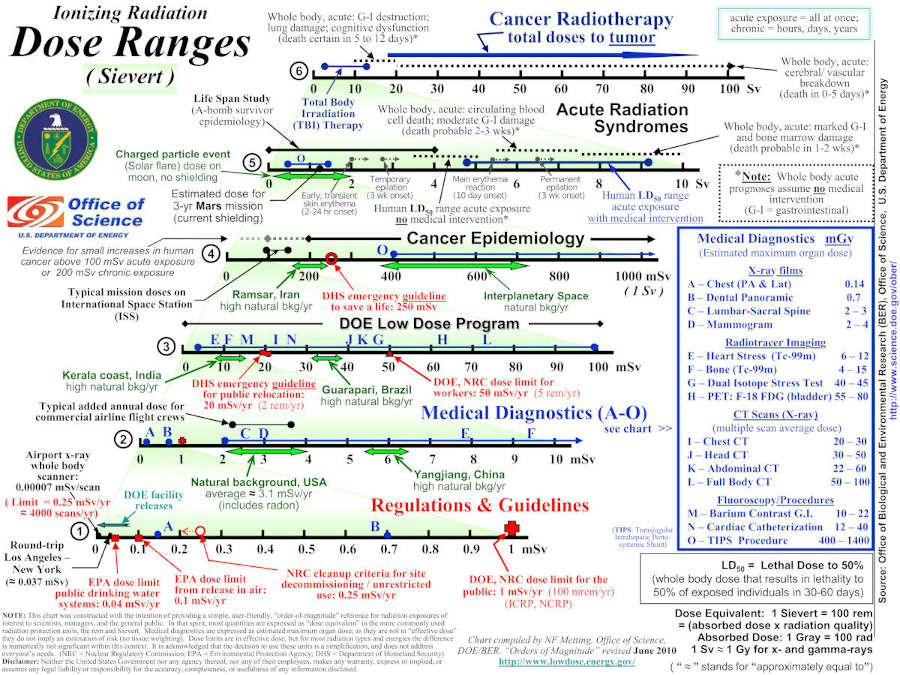
This info-sheet contains a wonderful description of radiation dose units and relative values. Our dose page contains more discussion.
Common sources of radiation
Radiation is all around us. The specifics of where it comes from in daily life are shown on this chart from the EPA:

Natural radiation
About half of our daily dose comes from completely natural sources.
Radon gas
This natural occurring gas comes from soil and is found throughout the world. It emits alpha particles, and can therefore damage DNA and lead to cancer if inhaled. The EPA recommends you check your house for radon gas.
Cosmic rays
Cosmic rays are energetic particles that originate outside of earth, in the sun, distant stars, galaxies, and supernovae. Most of these are protons. The atmosphere shields us from most cosmic rays, but during air travel, one will accumulate much higher dose. Don’t believe it? Check out our Radiation on Flights page.
“Man-made” radiation
The rest comes from synthetic radiation, which mostly comes from medical procedures. Nuclear power dose to the public is included in the ‘Industrial’ sliver in the figure above, representing <0.2% of our total dose.
Medical procedures
The vast majority of our man-made radiation is from medical procedures. Ionizing radiation is used to see into the body to help doctors figure out what to do. In some cases, strong focused beams of radiation are used to kill certain out-of-control cancer cells that cannot safely be excised by surgery.
Coal-burning power plants
Coal is an impure fuel, and it usually contains 1.3 ppm of uranium and 3.4 ppm of thorium (not to mention arsenic, mercury, and sulfur). When coal burns, these isotopes are emitted into the atmosphere, where they enter our ecosystem. This leads to the astounding fact that the population effective dose equivalent from coal plants is 100 times that from nuclear plants.
Nuclear weapon detonations
The hundreds of atmospheric nuclear weapons tests that occurred before they were banned by the 1963 Limited Test Ban Treaty left long-lived radioisotopes in the atmosphere. Some of these are still in the atmosphere and account for a tiny fraction of our daily dose.
Smoke detectors

Smoke detectors make use of the isotope Americium-241. This isotope emits alpha-particles at energies up to 5.4 MeV. The energetic alpha particles are used to ionize air. Once the air is ionized, a small current runs through it. When smoke enters the chamber, the current experiences an increase in resistance and a circuit sounds the alarm.
What exactly is nuclear radiation?
There are several types of particles or waves that may shoot out of a radioactive nucleus. Alpha particles, beta particles, gamma rays, and neutrons are the most common forms of ionizing (i.e. dangerous) radiation.
Alpha Particles
Named alpha because they were the first to be discovered, these particles are made up of 2 protons and 2 neutrons: the helium nucleus. Often, large atoms decay by emitting an energetic alpha particle. These particles are relatively large and positively charged, and therefore do not penetrate through matter very well. A thin piece of paper can stop almost any alpha particle. However, the particles cause extreme damage of materials that they stop in by displacing atoms as they slow. Paper under sustained alpha-irradiation would degrade.
Beta Particles
Beta particles are energetic electrons that are emitted from the nucleus. They are born when a neutron decays to a proton. Since neutrons are neutral particles and protons are positive, conservation of charge requires a negatively charged electron to be emitted. Some isotopes decay by converting a proton to a neutron, thus emitting a positron (an anti-electron). These particles can penetrate matter more than can alpha particles, and it takes a small aluminum plate to stop most beta particles.
Gamma rays
Gamma rays are photons that are emitted from the nucleus. Often an atom in an excited state will de-excite by emitting a gamma ray. Gamma rays are similar to light waves and x-rays, except they are usually much higher frequency and consequently, more energetic. This radiation has no charge, and can penetrate most matter easily, requiring lead bricks for shielding.
Analogy between radiation and heat
Radiation is a lot like heat, except you can’t feel it or see if there’s a fire. As everything around us is naturally a little bit warm, the world is also a little bit radioactive. Cosmic rays rain down from outer space 24/7. Radon gas has bubbled out of rocks for billions of years. The heat inside the earth is about 50% due to radioactive decay of primordial uranium and thorium.
No one gets hurt by things that are a little warm (up to 75°F/24°C), and no one gets injured by natural radiation (around 3 mSv/yr in the USA).
There are parts of the world that are hotter than normal (like Florida) and people live there just fine. Similarly, there are places in the world with higher natural radioactivity due to local geology, like Ramsar Iran with over 100 mSv/yr or Denver Colorado where cosmic rays give 2x more dose than this living at sea level. People live there just fine too.
Things that are very hot are hazardous. Boiling water can scald us. In controlled scenarios, hot things are still useful. We use controlled high heat to make steel and concrete, to cook, to do pottery. Doctors can use high heat in a very localized part of our skin to cauterize a wart.
Similarly, we can use high-dose radiation for many useful things. X-rays provide medical diagnostics that save lives. Radioactive isotopes have powered deep-sea science experiments, mars rovers, space probes, and pacemakers in hearts. Ionizing radiation was used to discover the double-helix nature of our own DNA. Nuclear power plants produce more than half of the carbon-free electricity in the USA, powering millions of homes. Doctors regularly use radiation in localized parts of our bodies to kill cancerous tumors and save lives.
Being engulfed in the flames of a fire can maim and kill us. We use fire code, flame retardants, fire alarms, fire fighters, and hospitals to defend against this hazard. Similarly, whole-body radiation doses above 4000 mSv of radiation can maim and kill. We use regulations, safety systems, radiation detectors, health physicists, and hospitals to defend against these analogous hazards.
Similarly, the dose rate that will be caused by the proposed release of Fukushima water is 0.0006 mSv/yr. This is over 1000x less than natural background. It is safe.
Video of radiation detection
Watch the a video of a few of us detecting radiation from household items.
Math of radioactive decay
If you’re looking for math, see the math behind radioactive decay page.
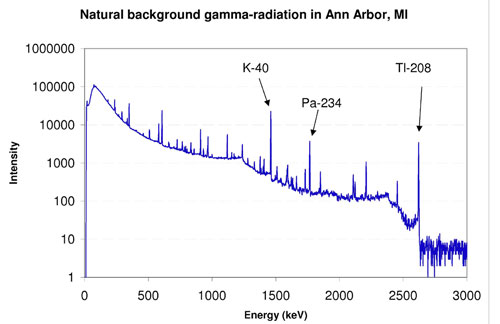
Background readings in Ann Arbor, MI
For a class in 2005, with no radioactive sources within range, we measured a long (30 minutes +) reading with a high-purity germanium (HPGe) gamma-ray detector system. We then identified the source of each peak. The spectrum is shown in the figure. Click it for the identifications. HPGe detectors are known for excellent resolutions, and as you can see, many peaks are clearly visible. Each one represents a specific nuclear reaction. Some major gamma-rays are highlighted on the figure. Thallium-208 is a decay-product of Thorium-232, which is naturally present in soil. Protactinium-234 results from the natural alpha-decay of Uranium-238. Potassium-40 is found all around, including in bananas and in salt-substitutes at the grocery store.
References and further reading
- Wikipedia, “Nuclear weapons testing”
- EPA Radon Guide
- Commercial air flight dose
- Mara Hvistendahl, "Coal Ash Is More Radioactive than Nuclear Waste," Scientific American (2007).
- Energy Information Administration - Spent Nuclear Fuel
- Center for Disease Control and Prevention: Radioisotope Brief: Cesium-137