What is an isotope?
Elements are your basic chemical building blocks. They include things like hydrogen, oxygen, sodium, magnesium, iron, titanium…, anything on the periodic table of the elements. Each element on the periodic table has a different number of protons in its atomic nucleus (its dense center). Each element has a few varieties with the same number of protons, but different numbers of neutrons in the nucleus. All isotopes of a particular element act chemically-identically to each other. Figure 1 shows the periodic table of the elements. Each element listed has many (between 2 and 20+) isotopes.

Figure 1. The Periodic Table of the Elements
The arrow points to Iron (Fe), which has a few isotopes shown in Figure 2.

Figure 2.The isotopes of Iron
Make sense? Great. One particularly relevant set of isotopes acting chemically similar but neutronically different are those of the element Uranium, shown below in Figure 3.

Figure 3.The isotopes of Uranium
Enrichment
Now that you know what isotopes are, you can understand exactly what enrichment is.
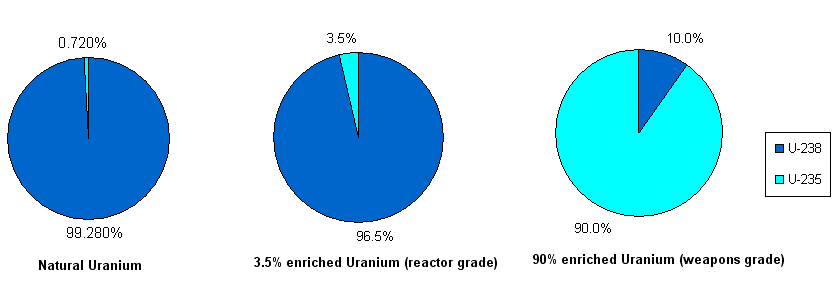
Natural Uranium is made up of 99.2745% U-238 and 0.720% U-235, with trace amounts of U-234. While U-238 usually stays together in a neutron field, U-235 readily splits, or fissions, in the presence of neutrons, releasing huge amounts of energy. This energy runs nuclear reactors and nuclear weapons alike. To create a chain reaction, you must enrich natural Uranium to contain more U-235. A typical nuclear reactor requires about 3.5% U-235, whereas a nuclear weapon requires more than 90% U-235. See figure below.
Try our interactive enrichment calculator
Enrichment is a very difficult process, as the mass difference between each isotope is minuscule. The US developed enrichment at Oak Ridge during World War II as part of the Manhattan Project. The newsworthy item about it is that anyone who can enrich can create highly-enriched uranium, a good material with which to make nuclear weapons. Countries that want to have their own nuclear fuel manufacturing capabilities argue that they need enrichment plants, but opponents argue that they are just looking to produce nuclear weapons.
Grammar alert: Isotopes vs. Nuclides
Isotope and nuclide are closely related terms. When one speaks of isotopes, they are referring to the set of nuclides that have the same number of protons. Nuclide is a more general term, referring to a nuclear species that may or may not be isotopes of a single element. Examples:
- “U-235 is my favorite isotope of Uranium.”
- “Cm-244, Pu-241, and Am-242m are lesser known fissile nuclides.”
Many people use them interchangeably, including experts in the field. Just read the MCNP manual!
